Cyclopharm (ASX:CYC)
Our investment strategy for Cyclopharm (ASX:CYC)
- Buy CYC up to $2.20.
- Our minimum target price for CYC is $3.00.
- Use a stop loss at $2.25 (update on 14 September from $1.20).
Disclosure: Pitt Street Research/Stocks Down Under directors own shares in Cyclopharm.
Cyclopharm in a nutshell
- Cyclopharm markets Technegas, a radiopharmaceutical product for functional lung ventilation imaging.
- The product is used around the world (in over 60 countries), but has yet to gain FDA approval, which would grant it access to the world’s biggest healthcare market.
- The company just resubmitted its application for FDA approval. This approval is expected within the next 6 months.
- Cyclopharm expects to grow sales significantly once it can market in the US.
Watch our recent interview with Cyclpharm’s CEO
Who is Cyclopharm?
Cyclopharm is a nuclear medicine company operating in the field of diagnostic imaging. Cyclopharm’s core radiopharmaceutical product, Technegas, represents the standard-of-care in most markets around the world for functional lung ventilation imaging. It is inhaled by the patient, acting as a replica of how ventilation is performed by the lungs, allowing a gamma camera to create an image of the lungs that can reveal potential diseases.
Technegas is the basis of an established business that sees the product sold in more than 60 countries with $23.2m in revenue in FY22 (calendar year) versus $17.7m in FY21.
Cyclopharm was not profitable in FY22, recording a net loss after tax of $6.1m, but expects that sales of Technegas post FDA approval can quickly lift the company to breakeven and beyond.
No approval in the US … yet
The one major market in the world in which Technegas is not approved yet is the US, which is the largest in the world (both for healthcare generally and for nuclear medicine). FDA approval of Technegas is expected by the end of September 2023 at the latest, after an earlier application was rejected by the Agency in mid-2021. On 30 March, CYC announced it resubmitted its application for FDA approval.
There is reason to be optimistic that FDA approval will finally happen this year, helped by strong support in the US medical community, the FDA’s visit to CYC’s Australian headquarters in the middle of pandemic travel restrictions and the fact that the early rejection (in the form of a ‘Complete Response Letter’) had to do with manufacturing data rather than clinical data. Cyclopharm submitted its case to the FDA at the end of March and the maximum period for the FDA to respond is 6 months.
In other words, there don’t seem to be any open questions as to the product itself.
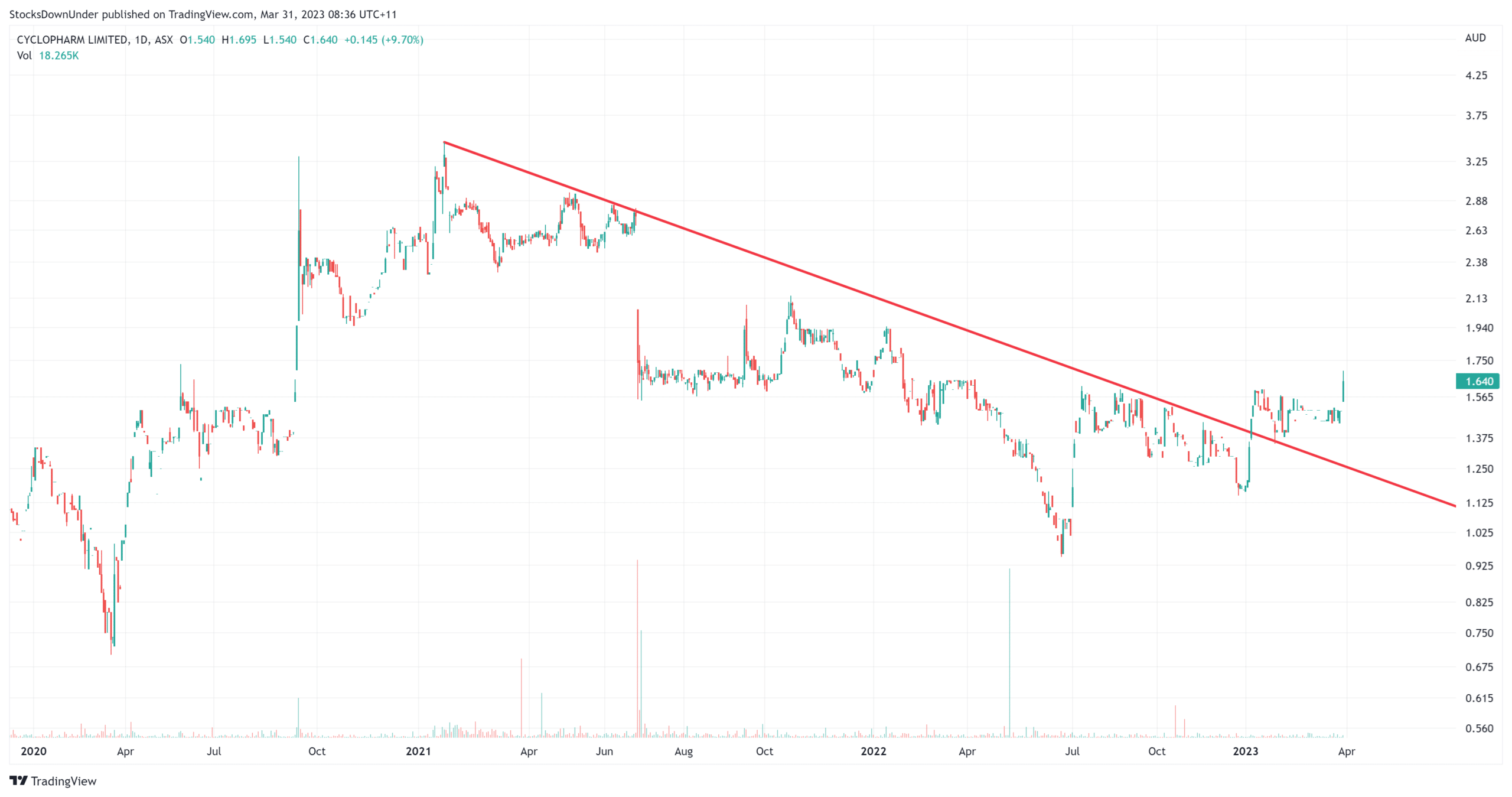
Cyclopharm (ASX:CYC) share price, log scale (Source: Tradingview)
Substantial addressable markets (now and in the future)
Technegas is currently used to diagnose Pulmonary Embolism, that is, potentially life-threatening blood clots in the arteries of the lungs. We estimate that market to be worth US$200m worldwide. But Cyclopharm has potential to expand Technegas to new applications that are much larger than Pulmonary Embolism, including, Chronic Obstructive Pulmonary Disease (COPD), but also asthma and Long Covid.
Cyclopharm believes that there is potentially an additional US$900m market opportunity just in the United States for Technegas in these follow-on indications.
Our investment thesis for Cyclopharm
- Cyclopharm has an established global business. Cyclopharm sells Technegas in over 60 countries and has witnessed significant growth in revenues as of late. Cyclopharm, which is a December year-end company, made $23.2m in revenue in FY22, up 31% from FY21.
- Cyclopharm has a market-leading product. Before Technegas the standard of care was CT Pulmonary Angiogram (CTPA). Technegas has sensitivity, specificity and accuracy at least equivalent to CTPA, but a 27x to 36x lower radiation dose.
- FDA approval is looming for Cyclopharm’s Technegas product, potentially in the next 6 months. Investors may be sceptical of Cyclopharm getting FDA approval, because the company has been trying for several years and was pushed back as recently as June 2021 when the FDA requested additional information related to the manufacturing of the product. There are several positive signs that Cyclopharm could be more fortunate this time around, including the FDA coming out to Australia to observe Technegas in action, even amidst pandemic travel restrictions in 2021. There is also strong support in the US medical community for CYC. We note that despite requesting further information, the regulator did not reject Technegas outright in June 2021, even though the share price reaction would suggest so. However, as always with FDA approvals, there is a chance that approval in the US may ultimately not be granted. We estimate that there is a 90% chance CYC will ultimately get FDA approval for Technegas.
- US approval quickly gets the company to breakeven. Cyclopharm was not profitable in FY22, recording a net loss after tax of $6.6m, but expects that sales of Technegas post FDA approval can quickly lift the company to breakeven and beyond.
- The US market is worth potentially US$90m to Cyclopharm in the near term, since this is what is currently spent on nuclear medicine to diagnose Pulmonary Embolism and Technegas would represent a safer alternative.
- Making progress in its major markets. CYC recorded growth in all of its markets except Europe in CY22. We note that Canada recorded the strongest growth of any specific market – CY22 sales in the country were worth A$2.96m that year, up 21% from CY21 and 68% from CY20. We believe the willingness of Canadian doctors to embrace Technegas bodes well for the product’s reception in the US from this year should the FDA say yes to the product.
- There is significant future potential in Technegas beyond diagnosis of Pulmonary Embolism. Currently, the company’s primary use for Technegas is Pulmonary Embolism, but CYC is investing in clinical trials to examine additional indications, including long-COVID, asthma and Chronic Obstructive Pulmonary Disease (COPD), all of which would be larger markets than PE. COPD alone is roughly 30x the size of the PE market.
- Cyclopharm is currently gathering data for the follow-on indications, with six clinical studies now being sponsored by the company, two in Australia and four in Canada.
- Cyclopharm has a strong balance sheet and pays dividends. The company has $20.3m in cash as at December 2022 and has no debt. It is also a dividend payer (paying 1c a share in FY22). We believe investors can be confident that it will not need a capital raising any time soon.
- Cyclopharm has multiple revenue streams. It does not just provide consumables, but earns revenue through after-sales services as well. As a provider both of the drug and the equipment, it has a natural moat. It also has a third-party distribution business that is making an increasingly material contribution to the business – it doubled its revenues in FY22.
- Technegas enjoys strong gross margins, at 72% in FY22. The kind of pricing the product enjoys can help fund more clinical work as well as drive a stronger global sales effort.
- Cyclopharm is substantially undervalued, on our numbers. If and when Cyclopharm gets US FDA approval for Technegas, we believe CYC should be valued above $3.00 per share, which signals substantial upside to the current share price. A March 2022 research report published by our parent company Pitt Street Research established a valuation range of $2.74-$3.33. That report is available at pittstreetresearch.com. Our valuation of the company without the US, however, is just $1.88-$2.27, still representing upside to the current share price.
- Cyclopharm has strong leadership. CEO James McBrayer brings a background in the radiopharmaceutical industry. Backing McBrayer is a board that has recently been renewed with the appointments of Kevin Barrow (formerly Managing Director at Philips Australia and New Zealand) and Professor Greg King (a respiratory physiologist at Sydney’s Royal North Shore Hospital).
Why has Cyclopharm underperformed since June 2021
- January 2021 was when the FDA sent its Complete Response Letter for Technegas.
- 2022 was a bad year for healthcare stocks after the valuations to which many companies had gone in 2020 and 2021. Cyclopharm was caught up in this bear market.
- Some investors may have lost patience with the 18-24 month delay for US Technegas approval.
$3.00 is our initial price target
- Cyclopharm is currently valued at 4.6x EV/Sales for FY24.
- As we noted above, we have modelled Cyclopharm and have come up with an initial $3.00 price target.
- $3.00 represents an EV/Sales multiple for CY24 of 7.7x, which is reasonable for high growth medical device plays.
- On an EV/EBITDA basis, the stock is currently valued at 36.6x FY24. Although this may appear high, it currently doesn’t include any US sales.
- Additionally, we see potential for the stock to go substantially higher than $3 in the longer term, once the value of the newer applications starts to be priced in by the market.
Catalysts for the re-rate of Cyclopharm
We see three main catalysts to prompt a re-rating of the stock:
- The single biggest share price catalyst will be FDA approval, expected by late September 2023 at the latest.
- Strong sales growth in other regions in the meantime. We’ll know more about that at the 1HY23 results announcement in July/August.
- A return to favour of Life Sciences companies, after a bad 2022, now that the interest rate outlook has changed almost overnight following the collapse of Silicon Valley Bank and a few others in the US.
Risks
- Failure to get FDA approval or any additional requests for information from the FDA (even prior to a final decision) will trigger a strong selloff, in our view.
- A return to COVID-19 restrictions, thereby impeding face-to-face sales meetings.
- Labour shortages in the healthcare industry.
- Failure of clinical trials that examine other indications for Technegas.
- Further turbulence on financial markets in 2023 that would affect healthcare stocks and their valuations.
UPDATE 2 October 2023: Cyclopharm receives FDA approval for Technegas
Well, it has finally arrived…FDA approval for Technegas! This morning Cyclopharm announced it received approval after initially having been knocked back by the FDA in 2021. Read the fulkl announcement here.
This approval opens up the vast US market, initially valued at US$180m, just for Pulmonary Embolism. But Cyclopharm received approval for broad use of the product, which means it will be able to address the markets for Chronic Obstructive Pulmonary Disease, Asthma, long COVID and lung cancer as well. In one of the reports published by Pitt Street Research, these markets were estimated to be ~30x larger than the Pulmonary Embolism market.
Muted market reaction so far
The initial market response took the share price up to $3.15 before it fell back below $3.00 (up ~4.5%). We think this muted response has to do with the public holiday in certain states and, consequently, low trading volumes across the board.
We expect CYC to go up substantially once investors return to their screens starting tomorrow. We’re up 83% on CYC 31 March 2023 and we’ll stick with it a bit longer in anticipation of prices substantially higher than $3.00.
Disclosure: Pitt Street Research/Stocks Down Under directors own shares in CYC.