Rhythm Biosciences (ASX:RHY): How and why did it all go wrong in 2023 and is there any way back?

Rhythm Biosciences (ASX:RHY) is back to square one after a rollercoaster 3 years. Where did it all go wrong, and is there a way back?
All about Rhythm Biosciences
Your analyst first came across this company in September 2019, right in the middle of his journalist days. The context was a ‘gathering over sandwiches’ with CEO Glenn Gilbert. For context, the most such gathering I attended was a 3-course lunch with Opthea (ASX:OPT), just 2 days after its successful Phase 2 trial results for its wet-AMD treatment. The food wasn’t as glamorous with Rhythm, or perhaps it shouldn’t have been given the company’s focus, although the opportunity looked compelling.
This was not the first or last gathering where I heard a company talking up its product, but the product Rhythm had actually was a good one. The only question was, whether or not it could execute. Back then, there was a large question mark. In 2021 and 2022, it seemed like everything was on track and that this company was and would continue to deliver. By the end of 2023, all the doubts are back again.
Rhythm Biosciences has been developing ColoSTAT, a simple blood-based test for the efficient detection of colorectal cancer at all stages. It was pioneered at the CSIRO in 2003 and exclusively licensed to RHY in 2017. Colorectal cancer is the third most common cancer in the world and can be treated if detected early.
To this end, Australian government has a formal screening program involving faecal screening, but this is not highly taken up by the population and is not as effective – it only detects blood in the stool, just one of many definitive bowel cancer biomarkers. Even when people are screened and advised for a colonoscopy, only 4% of these prove positive.
The rollercoaster journey up
Rhythm listed in late 2017 and began with isolating the antibodies and proteins to develop he reagents to identify the relevant biomarkers. A long and ardous process that many investors, especially of the retail kind, lack patience for. After two and a half quiet years, the company successfully validated a key biomarker in April 2020. Thereafter it conducted two clinical studies and made initial efforts to obtain regulatory approval. By this time, it had bought onboard Otto Buttula as chairman, a man who in a previous life developed IWL and sold it to the Commonwealth Bank for $370 million. All companies will tell you they have experienced management…but we think a company couldn’t go much further to back up its claims than to hire someone with a resume like his.
RHY obtained CE Mark approval in late 2021 (for Europe) and then sought to obtain approval in Australia. Confident off the back of its success, it advanced platform expansion with other cancers, particularly lung cancer.
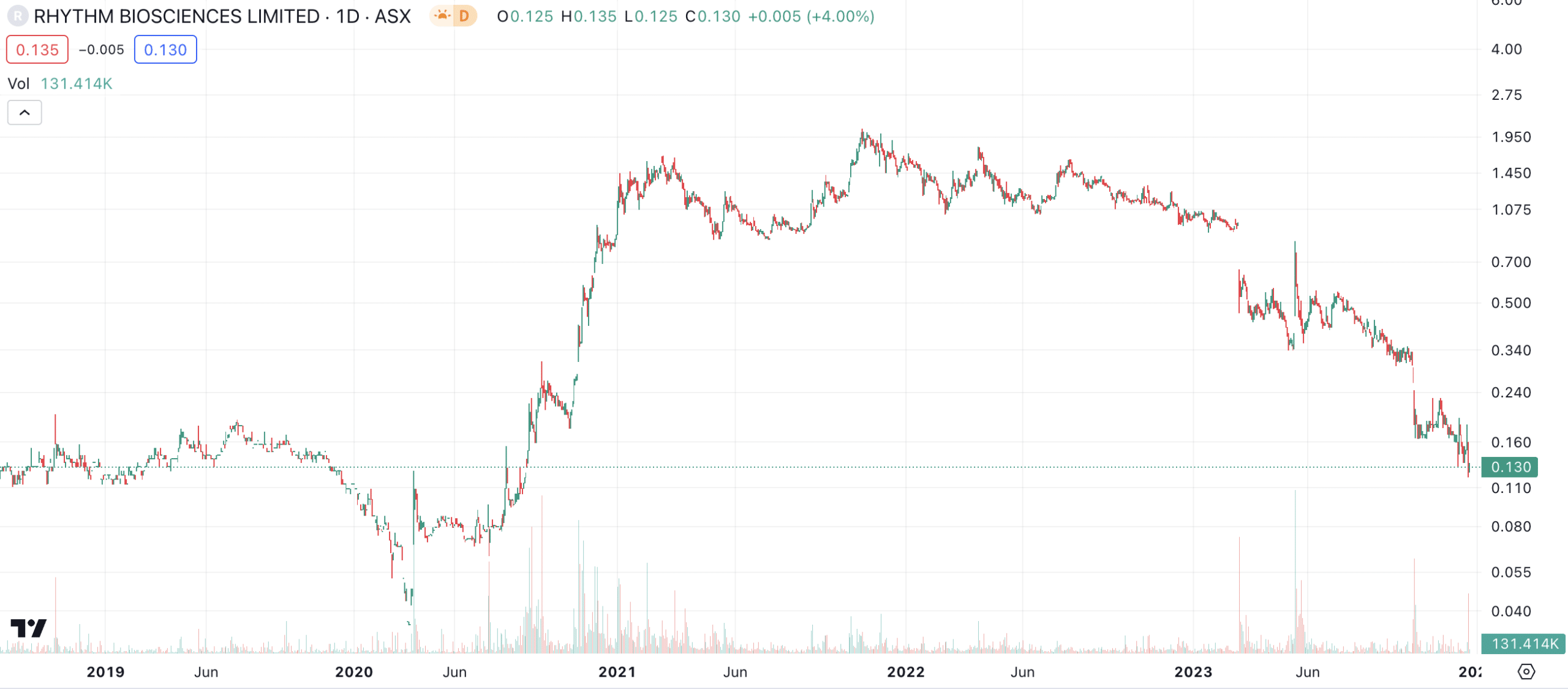
Rhythm Biosciences (ASX:RHY) share price chart, log scale (Source: TradingView)
What goes up comes down
In September 2022, the first warning sign can with a share sale by Otto Buttula. He sold 5m shares at $1.30 each to an institutional fund manager that was not formally disclosed (the AFR reported that the fund manager was Fidelity although this has not been confirmed). We’ll leave you to do the maths on how much the sale was was. There are a few ways companies use to defend director share sales like paying a tax bill, that institutional fundies wanted in or that too much of the director’s portfolio was in the stock. Rhythm chose the latter two argued those shares were 95.9% of the total assets and that the total package was only 17% of so of his holdings in the company.
The TGA application took longer than expected, so much so that by March 2023, Rhythm opted to withdraw the submission. Glenn Gilbert left around that point while Buttula has committed to gradually relinquish his duties over time as a new CEO is found and a subsequent handover period commences.
It was not an entirely a wasted year for the company, although it is back to square one. Its biomarkers for breast cancer had good results in a preliminary assessment, although the company was back to pre-clinical when it could easily have been in the commercialisation phase. It told investors it was preparing to submit a revised TGA submission, although it is anyone’s guess as to how successful it will be. Although it had CE Mark approval, the company faced the challenge of redesigning its test kits to be compliant with IVDR standards by May 2026.
We also got to hear the TGA’s side of the story, thanks to documents released under FOI Act. It that outlined there were issues with the submission and requested more information. The TGA was not satisfied with Rhythm’s response to that request and sought even more.
Last week the company released the results of a strategic review. The currently produced test kits will only be used for Research purposes only. It is now looking to produce kits to new IVDR standards going forward.
This means the company is essentially back to square one and has to do all the hard work again. As you’d expect, there will inevitably be significant cost cuts going forward.
Is there hope left?
There could well be, but it will take a long time for investors to trust the company again given how it made so much progress, only to see it all washed away.
What are the Best ASX Stocks to invest in right now?
Check our buy/sell tips

Blog Categories
Get Our Top 5 ASX Stocks for FY26
Recent Posts
How Nuclear Energy Became the World’s Most Feared Energy Source
Why Nuclear Energy Still Scares Us and What Really Went Wrong Many investors may remember periods when nuclear energy captured…
Dateline Resources (ASX:DTR) From 60c Highs to Hard Lessons
A Rare Earth Story the Market Loved Then Questioned For investors who have followed Dateline Resources (ASX:DTR), the past year…
Light and Wonder Surges 16% as A$190M Settlement Clears Major Legal Overhang
The Legal Overhang Is Gone Light and Wonder (ASX:LNW) saw a sharp and positive market reaction this morning, with the…


