Here’s what you need to know about the TGA Special Access Scheme and why its a game changer for ASX biotechs
![]() Nick Sundich, September 16, 2024
Nick Sundich, September 16, 2024
For ASX biotechs wishing to commercialise drugs in Australia, the Therapeutic Goods Administration (TGA) Special Access Scheme is the next best thing to regulatory approval. Therapeutic goods need to be included on the Australian Register of Therapeutic Goods (ARTG), prior to being sold, imported or exported from Australia. However, the Special Access Scheme provides a way for drugs to be accessed prior to market approval and this has benefited several ASX healthcare companies.
What is the TGA Special Access Scheme and how does it work?
The TGA Special Access Scheme enables medical practitioners to prescribe a good that is not registered on the ARTG (also known as an unapproved therapeutic good) in special circumstances. This can be done for individual patients or for multiple patients with the same conditions. For the latter, a medical practitioner needs to become an Authorised Prescriber – someone who can prescribe the goods in question. But if it is an individual patient, medical practitioners can apply to use the SAS. Technically, the Authorised Prescriber is a separate scheme to the SAS, but the industry often alludes to both schemes as the SAS, because they are similar in their operation and intent – enabling medicines to get to patients earlier where there is a compelling need.
When making the decision to use these unapproved therapeutic goods, the TGA takes several factors into account including the product, the patients’ condition, the prescriber’s ability to practice medicine, as well as state and territory requirements. The practitioner assumes all the risks for the use of the product, including liability if there are adverse reactions.
There are 3 pathways under the scheme. Category A can only be accessed by medical practitioners whose patients are seriously ill with a condition from which death is reasonably likely to occur within a matter of months, or from which premature death is reasonably likely to occur in the absence of early treatment. Category C is used where there no threat to life, but there is an established history of use for those products. There are specified goods, indications and types of health practitioners authorised to supply them. Category B is for instances that do not fit either – where there is not a risk of mortal peril and there isn’t an established history of use. An approval letter from the TGA is required before goods can be accessed.
Recce Pharmaceuticals is one company to have benefited
During FY24, Recce Pharmaceuticals benefited from the scheme, enabling its RECCE® 327 (R327) drug anti-infective drug to be used in certain patients suffering from life-threatening bacterial infections, unable to be treated by existing antibiotic therapies. R327 is Recce’s flagship asset, currently in several clinical trials putting it to the test against antibiotic-resistant superbugs. Superbug is a term applied to bacteria that are resistant to conventional antibiotics, R327 counters this with a unique Mechanism of Action, working fast and continuing to work just as effectively with repeated use.
Recce has announced that a total of 6 patients were treated with R327 under the TGA’s SAS Category A. These patients suffered from infections that were not responding to conventional antibiotics and were categorised as seriously ill. Five patients (Patients A-E) received topical treatment of R327 (in a gel form), with all five patients responding well to treatment resulting in wound healing, and surgical intervention was averted. The remaining patient (Patient X) was treated for P. aeruginosa sinusitis, with samples taken post-R327 treatment showing no detectable signs of P. aeruginosa infection and no abnormalities.
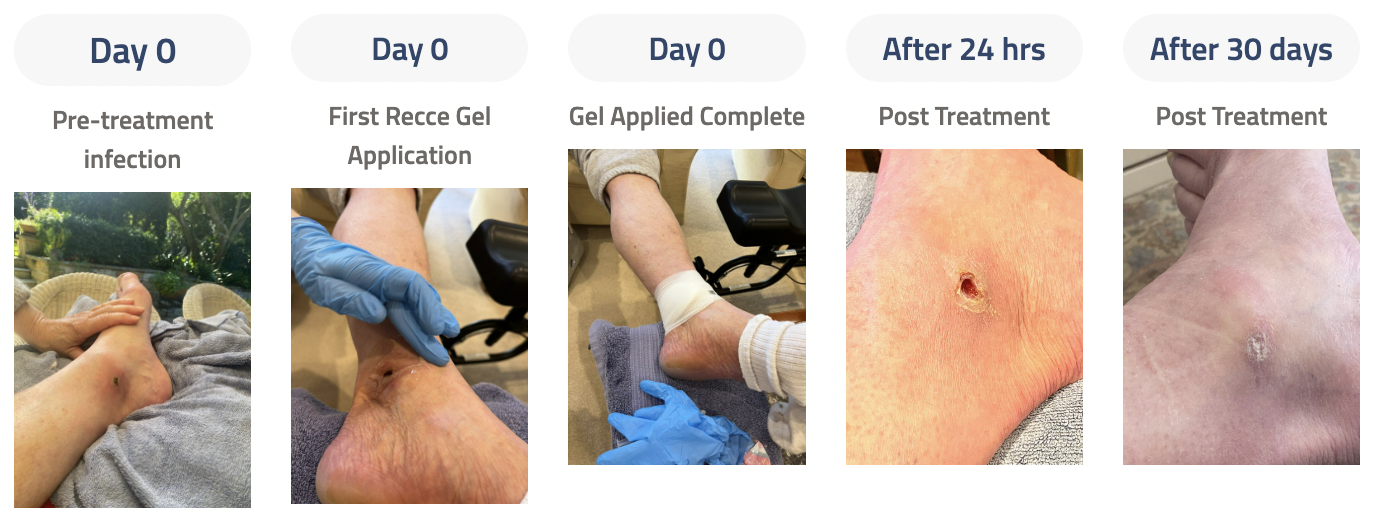
The recovery of Patient A, treated with R327 under the TGA’s SAS. (Source: Company)
Other stocks have benefited too
Plenty of other companies have benefited. In the last 5 years, medicinal cannabis stocks have been among the most prominent, given the hype around the sector. Examples have included Cann Group (ASX:CAN) and Wellnex Life (ASX:WNX). Among non-cannabis companies, LTR Pharma (ASX:LTR) is a fairly recent case study.
It was only a few weeks ago (in early August 2024) that LTR prescribed its erectile dysfunction nasal spray Spontan to its first patients. The Authorised Prescriber application was made by the Restorative Sexual Health Clinic (RSHC), which increased the likelihood of approval and gave credibility to the treatment’s introduction. Looking further back, Paradigm Biopharmaceuticals (ASX:PAR) was able to deliver its iPPS osteoarthritis treatment to patients when it was still in Phase 2. The successful results here complemented the clinical evidence in the trials the company conducted, enabling it to progress to Phase 3, where it is today.
The TGA Special Access Scheme is an important avenue for ASX biotech companies
Taking a drug to market is expensive and time-consuming, but the TGA’s SAS program can give investors an insight into the capabilities of new technologies while also providing innovative treatment options that can save patients lives. The scheme can provide anecdotal evidence of a drug’s safety and efficacy before eventual formal approval.
This is a sponsored article – sponsored by Recce Pharmaceuticals. Paradigm Biopharmaceuticals is a research client of Pitt Street Research, but did not sponsor this article.
What are the Best ASX Stocks to invest in right now?
Check our ASX stock buy/sell tips
Blog Categories
Get Our Top 5 ASX Stocks for FY25
Recent Posts
Apple’s iPhone Production in Focus: Is the Tariff Pause Enough to Ease the Pressure?
Apple, one of the largest and most influential tech companies in the world, is no stranger to the fluctuations of…
Why travel shares are getting slammed…and it is not for the reasons you may think
Just when ASX travel shares were out of the COVID-19 doldrums (in that some surpassed their pre-COVID highs), 2025 looks…
Capital Gains Tax on Stocks: Here’s what you need to know
Investors may be liable to pay Capital Gains Tax on Stocks, but may not know the nuances of how it…