FDA approval is hard to get as these 2 companies are finding out
![]() Nick Sundich, February 1, 2023
Nick Sundich, February 1, 2023
There are several ASX health companies that are aspiring to sell their medical devices or drugs in the USA. This involves getting the device or drug, or the plans for a clinical trial, approved by the FDA (Food and Drug Administration).
But it is easier said than done as shareholders of Island Pharmaceuticals (ASX:ILA) and Mesoblast (ASX:MSB) have been learning the hard way.
No time to do stock research, but you still want to invest?
Stocks Down Under Concierge gives you timely BUY and SELL alerts on ASX-listed stocks!
With price targets, buy ranges, stop loss levels and Sell alerts too.
GET A 3-MONTH FREE TRIAL TO CONCIERGE TODAY
Island Pharmaceuticals’ plans knocked back by the FDA
(ASX:ILA) is developing a drug it hopes can fight mosquito diseases, particularly Dengue. Just before Christmas, it made an Investigational New Drug (IND) application to the FDA, seeking permission to start the clinical trial. The company told shareholders it would expect an answer in 30 days.
This answer has come, but it was not the one shareholders wanted.
It wasn’t good news
The FDA did not approve the IND, instead placing it on Clinical Hold and telling the company that it would need to alter the protocol and the IND in order to advance the program.
Island told shareholders it was working with vendors and consultants to formulate the best plan. Shareholders are evidently not optimistic, as the 25% share price decline in the last fortnight suggests.
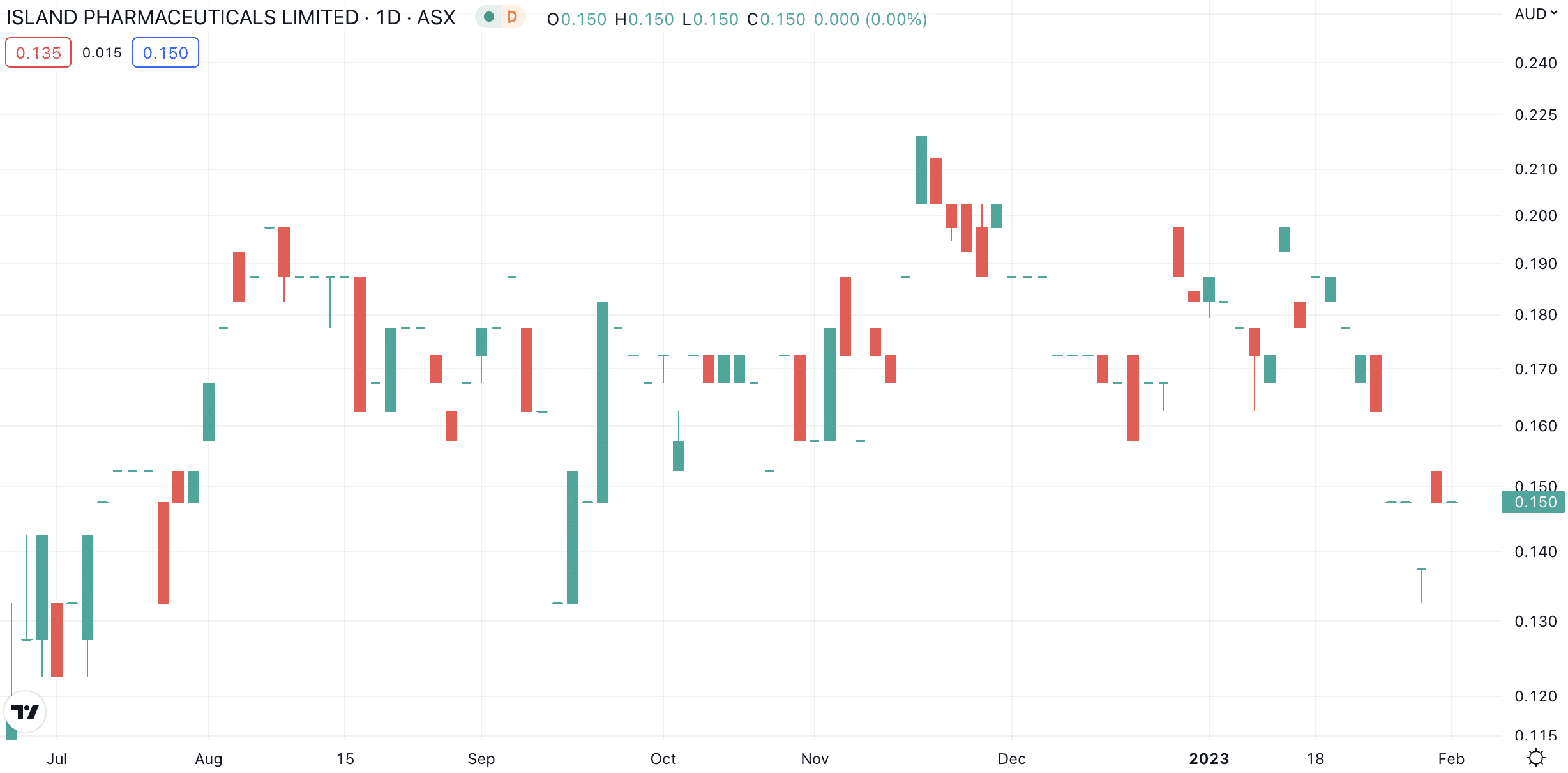
Island Pharmaceuticals (ASX:ILA) share price chart (Graph: TradingView)
Mesoblast is working on a resubmission too
Mesoblast is at a later stage to Island Pharmaceuticals, having already conducted clinical trials for its Remestemcel-L stem cell treatment.
The company is now trying to obtain FDA approval for remestemcel-L for steroid-refractory acute graft versus host disease. The company was first knocked back in September 2020 and it has been trying to obtain approval ever since.
Mesoblast has told shareholders that it made its case to the FDA once again and that it had addressed the FDA’s concerns. Investors will need to wait up to six months for the results, because this is the maximum period the FDA takes to review a submission. Oh well…more waiting.
No time to do stock research, but you still want to invest?
Stocks Down Under Concierge gives you timely BUY and SELL alerts on ASX-listed stocks!
With price targets, buy ranges, stop loss levels and Sell alerts too.
GET A 3-MONTH FREE TRIAL TO CONCIERGE TODAY
No credit card needed and the trial expires automatically.

Blog Categories
Get Our Top 5 ASX Stocks for FY25
Recent Posts
Your invitation to the Freelancer Investor Day
Your invitation to the Freelancer Investor Day Freelancer (ASX: FLN) is a Sydney-based company that has been the subject of…
Kamala Harris stocks: If Joe Biden’s VP wins the White House in 2024, which stocks will win?
With the US Presidential election now certain to be a Kamala Harris v Donald Trump showdown, we’ve looked at so-called…
South32 (ASX:S32): Is it the dark horse amongst ASX 200 miners or have cyclones and commodity prices hit it too hard?
South32 (ASX:S32) began life as a spinoff from BHP back in 2015, capitalised at $9bn. In mid-2024, it is capped…


