Telix Pharmaceuticals (ASX:TLX): It’s made ~A$1.7bn in revenue from Illucix, but here’s why the best is yet to come!

What would you have thought if you were told 5 years ago you would see Telix Pharmaceuticals as a successful story? It would have been possible believe back in those days, but it was still far from certain it would be the case. The recent example of Opthea shows biotech is a risky business and it has been a long journey to get to Illucix to market.
But having done that, the company has made over A$1.4bn in 2 and a half years from selling Illucix in the US market. Is there any more growth left in Telix? Let’s take a look.
All about Telix
Telix Pharmaceuticals is one of the few biotechs that has gone from a microcap clinical stage company to a fully commercial company. Telix’s flagship product is Illuccix, which is used for the imaging of prostate cancer and is FDA approved. It is working on a second product for imaging renal cancers, but for now, Illuccix is the primary source of its revenue.
Telix Pharmaceuticals listed on the ASX in 2017 at 65 per share, valuing it at $150m. At the time, it was the biggest biotech IPO in over 20 years (since CSL’s privatisation). But, Telix has grown nearly 40x larger, at $8.5bn as of April 22, 2025. It is up 78% in the last 12 months.
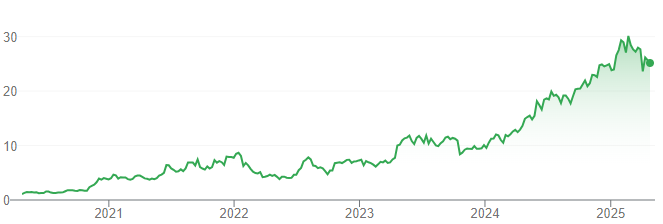
Telix Pharmaceuticals (ASX:TLX) share price chart, log scale (Source: TradingView)
Telix is now one of the largest radiopharmaceutical companies globally, trailing only behind giants such as Switzerland’s Novartis AG, Germany’s Bayer AG, and U.S.-based Lantheus. Yes, even though it has just one product.
Telix Pharmaceuticals taking more than four years post-listing to obtain approval for Illuccix depicts the difficulty of these steps. But its share price rise illustrates that if a company can overcome all the obstacles, the gains can be large.
Telix is going from strength to strength
Telix’s revenues have kept growing exponentially ever since Illuccix was commercialised. In the June quarter of CY22, it made A$22.5m in revenue, up 10x from the quarter before. The cash just kept coming in:
- A$55.3m in the September quarter of CY22
- A$78.2m in the December quarter of CY22
- A$100.1m in the March quarter of CY23
- A$120.7m in the June quarter of CY23
- A$133.6m in the September quarter of CY23
- A$148.1m in the December quarter of CY23
- A$175.0m in the March quarter of CY24
- A$189.0m in the June quarter of CY24
- A$201m in the September quarter of CY24, and
- A$218m in the December quarter of CY24.
- A$291m in the March quarter of CY25.
That all adds up to roughly A$1.7bn (Note: All these figures were at exchange rates at the time of reporting). The company expects $1.18-1.23bn in CY24, or US$770-800m. It expects R&D expenditure to be 20-25% of revenues. But this won’t just be from Illucix in the US.
The company is expecting to launch Illucix in Europe, having received approval in January 2025. Illucix has also been approved in the UK, Canada and Brazil, thus meaning it could be launched there in the coming years. And Telix has 3 new products planned to be launched.
Telix’s trinity of new products
- TLX007-CDx (Gozellix),
- TLX101-CDx (Pixclara) and,
- TLX250-CDx (Zircaix)
Gozellix’s New Drug Application was approved by the FDA in March 2025. This is in effect the next generation product after Illucix, with a longer shelf life, an extended distribution radius and the ability to serve more cameras (specifically, it can serve those not served by PSMA imaging providers).
Zircaiux is its kidney cancer imaging agent. It received a Priority review in launch and the FDA provided a ‘PDUFA date’ of August 27 this year. For those wondering, PDUFA alludes to the Prescription Drug User Fee Act and the PDUFA date is the term used to describethe date by which the FDA must respond to an application. The Priority Review came off the back of spectacular Phase 3 results which showed a sensitivity of 86%, a specificity of 87% and positive predictive value of 93% including in very small, difficult-to-detect lesions. In other words, it can detect cancers difficult to detect.
Pixclara is set to be approved any day now because its PDUFA date is April 26. It too is an agent that targets a brain cancer called glioma.
Telix also aspires to continue its clinical programs. The most important is the ProstACT Global Phase 3 prostate cancer therapy trial in the US, although there are some earlier stage trials too. Earlier in April, it reported preliminary results from a Phase 2 study of TLX101 in high-grade glioma.
It completed a number of M&A deals in 2024 including ARTMS, IsoTherapeutics and RLS to commence GMP production in 2025. These will all help the company with its production – all these companies had radiopharmaceutical technologies that will help Telix with its own production
Investors can be confident, but not complacent
Many investors have been concerned about the impact of Trump’s tariffs. The company has told investors it isn’t worried because pharmaceutical products are exempt from reciprocal tariffs, and many goods are made locally because radiopharmaceutical products have to be manufactured or radio-labelled close to the point of care.
It also does not rely on rare earth elements of the same kind utilised in semiconductor chains. Telix has also told investors it has not been notified of any changes to its timelines for regulatory applications notwithstanding cuts to the FDA.
There is one thing that has irked investors. In February, CEO Christian Behrenbruch sold 2m shares as $29.50 a pop, reaping A$59m. He still owns 6.3% of the company and has a 12-month escrow on them. Another 2m shares were sold by former director and co-founder Andreas Kluge.
Investors never like it when senior executives sell shares – especially when they reap 6 figures or more. However, Kluge is no longer a director and Behrenbruch’s sale was at least in part to pay a divorce settlement…there have been worse reasons/excuses company bosses have given for selling shares.
Telix is well-positioned
Telix is one of the few biotechs on the ASX with a commercialised product. It can inevitably gain more revenue as it continues commercialising Illuccix in the USA and elsewhere. And if it can commercialise other products in its pipeline, that could be further good news.
Nonetheless it remains to be seen for sure of Trump’s cuts to the FDA and his tariffs will impact the company in anyway. For now there seems to be not much of an impact (other than movements to its share price), but only time will tell.
As long as investors recognise the risks above, we think this company is one of the best-positioned biotech stocks on the ASX.
What are the Best ASX Stocks to invest in?
Check our buy/sell tips

Blog Categories
Get the Latest Insider Trades on ASX!
Recent Posts
Objective Corporation (ASX:OCL) is a superb ASX 200 tech stock
Objective Corporation (ASX:OCL) is one of a kind. There are few companies with a 2-decade listed life without raising a cent…
AI-Media Technologies (ASX:AIM): Investors at panicking that it’ll be a victim of AI
AI-Media Technologies (ASX:AIM) is not the only ASX stock with investors panicking that AI will make it go the way…
Geopolitics, AI, and Energy, The Three Pillars of Investment Growth in 2026
Investing right now feels riskier than ever – messy geopolitics, the AI boom, and power shortages are all piling on.…


